Les patients atteints de coronaropathie stable et de diabète sucré n’ayant subi ni infarctus du myocarde ni accident vasculaire cérébral courent un risque élevé d’événements cardiovasculaires. Cette étude de membres du consortium iVASC a cherché a comprendre si l’ajout de ticagrelor à l’aspirine améliore les résultats chez cette population.
P.G. Steg, D.L. Bhatt, T. Simon, K. Fox, S.R. Mehta, R.A. Harrington, C. Held, M. Andersson, A. Himmelmann, W. Ridderstråle, M. Leonsson‑Zachrisson, Y. Liu, G. Opolski, D. Zateyshchikov, J. Ge, J.C. Nicolau, R. Corbalán, J.H. Cornel, P. Widimský, and L.A. Leiter, for the THEMIS Steering Committee and Investigators
Ticagrelor in Patients with Stable Coronary Disease and Diabetes
N Engl J Med 2019. PubMed
BACKGROUND
Patients with stable coronary artery disease and diabetes mellitus who have not had a myocardial infarction or stroke are at high risk for cardiovascular events. Whether adding ticagrelor to aspirin improves outcomes in this population is unclear.
METHODS
In this randomized, double-blind trial, we assigned patients who were 50 years of age or older and who had stable coronary artery disease and type 2 diabetes mel- litus to receive either ticagrelor plus aspirin or placebo plus aspirin. Patients with previous myocardial infarction or stroke were excluded. The primary efficacy out- come was a composite of cardiovascular death, myocardial infarction, or stroke. The primary safety outcome was major bleeding as defined by the Thrombolysis in Myocardial Infarction (TIMI) criteria.
RESULTS
A total of 19,220 patients underwent randomization. The median follow-up was
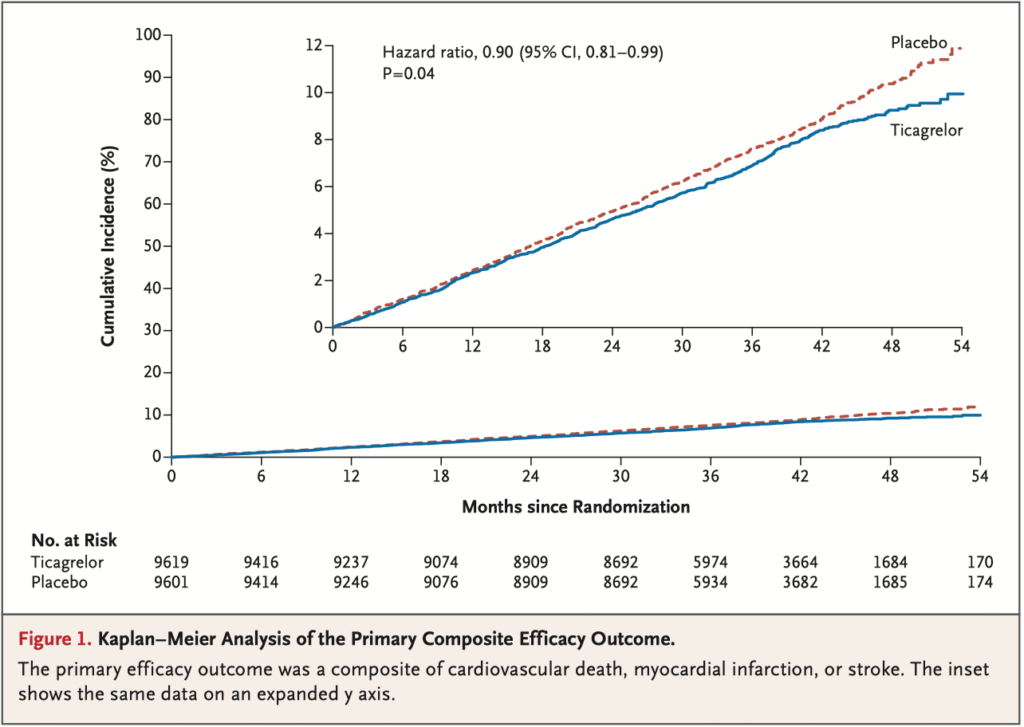
39.9 months. Permanent treatment discontinuation was more frequent with tica- grelor than placebo (34.5% vs. 25.4%). The incidence of ischemic cardiovascular events (the primary efficacy outcome) was lower in the ticagrelor group than in the placebo group (7.7% vs. 8.5%; hazard ratio, 0.90; 95% confidence interval [CI],
0.81 to 0.99; P = 0.04), whereas the incidence of TIMI major bleeding was higher (2.2% vs. 1.0%; hazard ratio, 2.32; 95% CI, 1.82 to 2.94; P<0.001), as was the in- cidence of intracranial hemorrhage (0.7% vs. 0.5%; hazard ratio, 1.71; 95% CI, 1.18 to 2.48; P = 0.005). There was no significant difference in the incidence of fatal bleeding (0.2% vs. 0.1%; hazard ratio, 1.90; 95% CI, 0.87 to 4.15; P = 0.11). The incidence of an exploratory composite outcome of irreversible harm (death from any cause, myocardial infarction, stroke, fatal bleeding, or intracranial hemor- rhage) was similar in the ticagrelor group and the placebo group (10.1% vs. 10.8%; hazard ratio, 0.93; 95% CI, 0.86 to 1.02).
CONCLUSIONS
In patients with stable coronary artery disease and diabetes without a history of myocardial infarction or stroke, those who received ticagrelor plus aspirin had a lower incidence of ischemic cardiovascular events but a higher incidence of major bleeding than those who received placebo plus aspirin. (Funded by AstraZeneca; THEMIS ClinicalTrials.gov number, NCT01991795.)